NHS patients with Alzheimer's WON'T access 'miracle' drugs as watchdog says they are 'too expensive'
Two of the first drugs proven to slow down Alzheimer's disease will be denied to NHS patients from this week - unless they pay to go private.
The National Institute for Health and Care Excellence (NICE) has refused the 'miracle' drugs lecanemab and donanemab for use on the NHS as they are too expensive to justify.
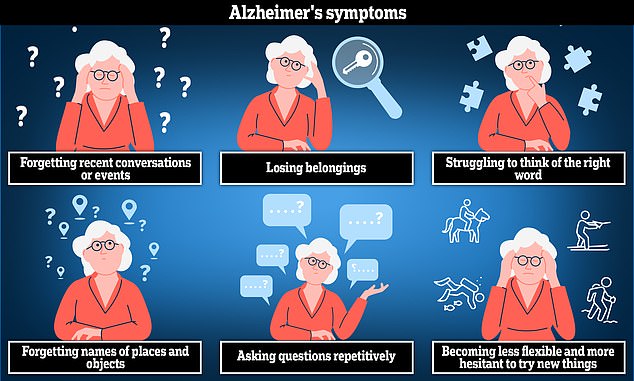
This means over 70,000 patients in England will be denied the 'game-changing' drugs, found to slow cognitive decline by an average of four to seven months, unless they can afford tens of thousands of pounds a year for private treatment.
After a positive drug trial in 2022, lecanemab (brand name Leqembi) made by Eisai, and donanemab (marketed as Kisunla), made by Eli Lilly, were proven to clear toxic amyloid protein from the brain and thus slow the underlying cause of dementia.
Campaigners hailed it as 'the beginning of the end' of Alzheimer's.
Iain Hartnell, Research Communications Officer at Alzheimer's Society , said: 'The MHRA’s approval of the first safe and effective Alzheimer’s disease treatment, shown to slow progression, is a defining moment for people with early-stage Alzheimer’s disease in the UK and a significant step towards a more hopeful future.'
Last year, both drugs were given drug licences in the UK by the Medicines and Healthcare products Regulatory Agency (MHRA).
But without NICE's recommendation, the groundbreaking medicine will only be available to those with private healthcare.

'It is the end of the road for these drugs on the NHS,' an insider told The Times .
Hilary Evans-Newton, the chief executive of Alzheimer's Research UK described the decision as 'deeply disappointing'.
She told the publication that it sends 'a damaging signal to the life sciences sector - undermining confidence in the UK as a leader of dementia research, clinical trials and innovation, with knock-on effects for patients and the wider economy'.
She added: 'These treatments are not perfect, and we recognise the challenges they pose around cost, delivery and safety. But scientific progress is incremental, and these drugs represent a vital foundation to build on.'
The drugs were shown in trials to slow the rate of decline for those with mild to moderate Alzheimer's by an average of four to seven months.
Lecanemab, for example, can slow the decline in memory and mental agility by 27 per cent in those with mild Alzheimer's taking the drug compared to people on the dummy drug.
The research team also found that the drug reduced loss of quality of life by up to 56 per cent, according to the Alzheimer's Society .
Importantly, the drug reduced the amount of amyloid protein present in the brain. Amyloid protein levels were also reduced in the blood and spinal fluid.
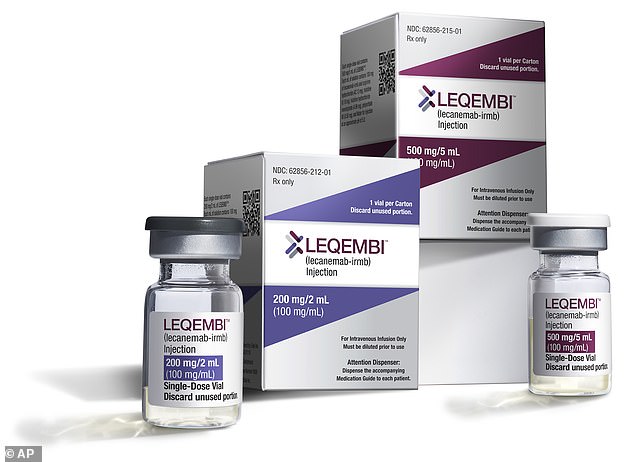
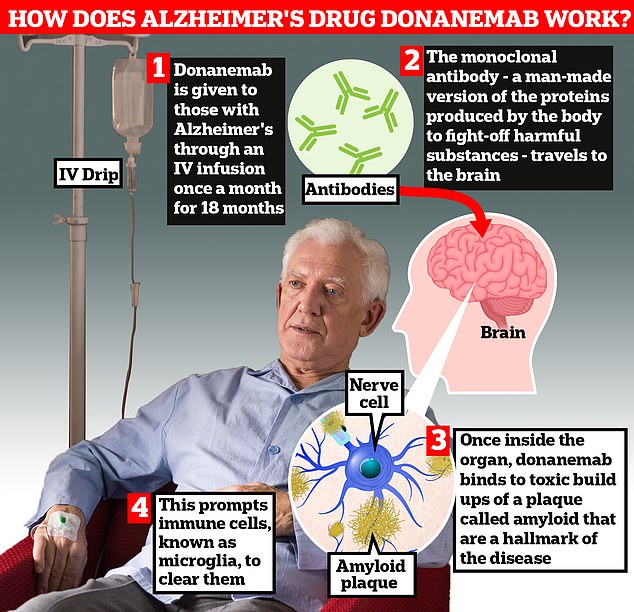
Lecanemab has already been given the green light in the EU, US, China, Japan, Hong Kong, South Korea and Israel.
It is estimated around 70,000 adults in England could eligible for the treatment if approved for use on the health service.
Alzheimer's is the most common cause of dementia in the UK. Around 982,000 are currently estimated to be living with dementia UK - although this figure is expected to skyrocket to 1.4million in 2040.
Alzheimer's Research UK analysis found 74,261 people died from dementia in 2022, compared to 69,178 a year earlier, making it the country's biggest killer.
Recent analysis by the Alzheimer's Society estimates the overall annual cost of the dementia to the UK is £42billion a year, with families bearing the brunt.
An ageing population means these costs — which include lost earnings of unpaid carers — are set to soar to £90billion in the next 15 years.
MailOnline has approached NICE for comment.
Read more
Posting Komentar untuk "NHS patients with Alzheimer's WON'T access 'miracle' drugs as watchdog says they are 'too expensive'"
Posting Komentar